Inflammatory bowel disease (IBD) affects over 2 million Americans. While advances in medical therapy have improved the care of IBD patients, many still suffer from refractory disease with significant morbidity. IBD includes two distinct clinical phenotypes called Crohn’s disease and ulcerative colitis. Although genetic polymorphisms correlate with disease susceptibility, environmental triggers including diet and intestinal bacteria are thought to play a central role in disease pathogenesis. While certain intestinal bacteria may act as opportunistic pathogens causing infection and inflammation, we and others have shown that microbiota function as a double-edged sword by simultaneously working to maintain homeostasis at mucosal surfaces. Thus, the major focus of our work is to define the cellular, molecular, and microbial regulation of intestinal barrier immunity and to develop novel diagnostic and therapeutic approaches for medically refractory IBD.
Post-doctoral fellowship positions available!
Please email Randy at ral2006@med.cornell.edu
Immunology and Mucosal Healing in IBD
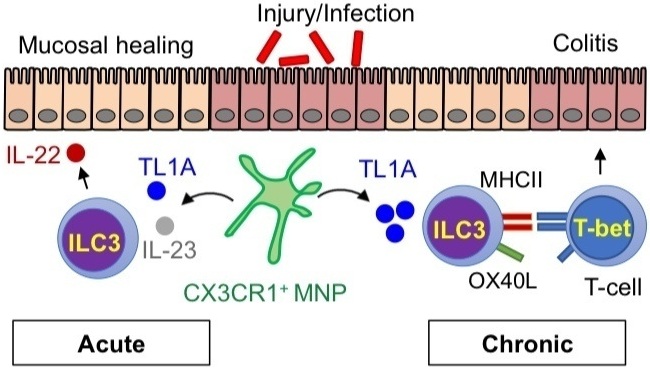
One part of our lab focuses on understanding the cellular and molecular mechanisms of homeostatic inhibition/induction by microbes within the intestine. Mononuclear phagocytes (MNPs) in the intestine which expresses the fractalkine receptor CX3CR1 play a critical role in this process. Under conditions of colitis, these MNPs expand in the lamina propria and activate innate lymphoid cells (called ILC3s) to produce the cytokine IL-22 and promote mucosal healing. Critical questions remain regarding the nature of the bacterial signals impacting these cells, the local and molecular signals regulating these cellular interaction, and the in vivo role in achieving mucosal healing.
The Microbiome of Extra-intestinal Manifestations
A second part of the lab focuses on microbial changes associated with extra-intestinal manifestations (EIMs) of IBD and their influence on mucosal and systemic immunity. In particular, seronegative spondyloarthritis (SpA) designates a group of diseases with overlapping genetic and clinical features in which the gut is the putative port of entry for microbial triggers of systemic cellular inflammation resulting in joint disease. By studying patients with IBD-associated SpA, we have a unique opportunity to understand the process by which luminal microbes shape systemic immune repertoires.
Click here to read more about our work on IBD and joint inflammation>
Translational Research at the Jill Roberts Center
We are committed to performing high-quality research with direct implications for improving the diagnostics and therapeutics in the treatment of IBD. To achieve this, the scientists at the Roberts Institute work seamlessly with the physicians at the Roberts Center to capture clinical data and biospecimens for research. This includes specific studies on the role for fecal microbiota transplantation in ulcerative colitis, medical therapy and the microbiome in Crohn's disease associated joint inflammation, and the role for new emerging therapy. We have also pioneered a unique, longitudinal biobank which enables us to provide a high resolution characterization of the intestinal immune system and discover new diagnostic and therapeutic targets of disease for all of our patients.